Button Mushrooms Vitamin D
Abstract
Background
/Objectives:Mushrooms contain very little or any vitamin D2 but are abundant in ergosterol, which can be converted into vitamin D2 by ultraviolet (UV) irradiation. Our objective was to investigate the bioavailability of vitamin D2 from vitamin D2-enhanced mushrooms by UV-B in humans, and comparing it with a vitamin D2 supplement.
Subjects/Methods:
Fresh mushrooms were irradiated with an UV-B dose of 1.5 J/cm2, increasing vitamin D2 content from <1 to 491 μg/100 g and made to an experimental soup. In this 5-week, single-blinded, randomized, placebo-controlled trial, 26 young subjects with serum 25-hydroxyvitamin D (25OHD) ⩽50 nmol/l were randomly assigned into three groups ((a) mushroom, (b) supplement and (c) placebo). They received during winter (a) 28 000 IU (700 μg) vitamin D2 via the experimental soup, or (b) 28 000 IU vitamin D2 via a supplement or (c) placebo, respectively.
Results:
After 2 weeks, serum 25OHD was significantly higher in the mushroom than in the placebo group (P=0.001). The serum 25OHD concentrations in the mushroom and supplement groups rose significantly and similarly over the study period by 3.9 nmol/l (95% confidence interval (95% CI): 2.9, 4.8) and by 4.7 nmol/l per week (95% CI: 3.8, 5.7), respectively.
Conclusions:
We are the first to demonstrate in humans that the bioavailability of vitamin D2 from vitamin D2-enhanced button mushrooms via UV-B irradiation was effective in improving vitamin D status and not different to a vitamin D2 supplement. This trial was registered at http://germanctr.de as DRKS00000195.
Introduction
Low vitamin D status, defined as a serum 25-hydroxyvitamin D (25OHD) concentration <50 nmol/l (Holick and Chen, 2008; Malabanan et al., 1998), is a public health issue prevalent worldwide (Chapuy et al., 1997; Hintzpeter et al., 2008; Tangpricha et al., 2002; van der Wielen et al., 1995), particularly in regions with a big seasonal shift in solar altitude, as the major source of vitamin D for humans is sunlight-induced cutaneous synthesis (Engelsen et al., 2005; Webb et al., 1988). Other criteria, like dark skin (Armas et al., 2007; Clemens et al., 1982), old age (Need et al., 1993) and immobility (Semba et al., 2000) further reduce the endogenous vitamin D synthesis. Moreover, few foods contain vitamin D in noteworthy concentrations; those that do are fish-liver oils, fatty fish and egg yolk. Furthermore, there is a wide variety of foods fortified with vitamin D across the world (Calvo et al., 2005), that is, dairy products, bread, and recently, orange juice (Biancuzzo et al., 2010; Holick et al., 1992; Natri et al., 2006).
Naturally, the vitamin D2 content of cultivated mushrooms is almost nil (<0.1 μg/100 g fresh weight) yet they are very rich in ergosterol (Jasinghe and Perera, 2005; Mattila et al., 2002). Ergosterol is the principal sterol in fungi, and several studies have reported that mushrooms can be greatly enhanced with vitamin D2 by ultraviolet (UV) irradiation, resembling the cutaneous synthesis of vitamin D3 in humans (Ko et al., 2008; Mau et al., 1998). The conversion rate of ergosterol to vitamin D2 under UV irradiation depends on the UV spectrum (UV-B or -C), irradiation dose, moisture content and the mushrooms' orientation toward the UV source (Jasinghe and Perera, 2005, 2006; Roberts et al., 2008).
Outila et al. (1999) were the first to demonstrate that vitamin D2 was well absorbed from lyophilized and homogenized mushrooms in humans. Jasinghe and Perera (2005, 2006) were the first to publish in vivo studies on the bioavailability of vitamin D2 from UV-irradiated mushrooms, showing as others (Koyyalamudi et al., 2009) that vitamin D2 from vitamin D2-enhanced mushrooms is well absorbed and metabolized in rodents and that it improves bone mineralization.
Recently the case history of a patient with vitamin D deficiency and secondary hyperparathyroidism was published, who refused to take supplements, but self-treated his deficiency by consuming mushrooms daily, which he had exposed to UV-B irradiation (Ozzard et al., 2008).
To the best of our knowledge, this is the first report on the bioavailability of vitamin D2 from UV-treated mushrooms in humans. Hence the primary objective of this randomized controlled trial was to demonstrate the possibility of improving the 25OHD status with this natural food source in terms of a higher serum 25OHD concentration in young adults with low 25OHD status 4 weeks after a weekly vitamin D2 dose of 28 000 IU (700 μg) compared with placebo. A secondary objective was to compare the bioavailability of vitamin D2 from UV-B treated mushrooms with a vitamin D2 supplement.
Subjects and methods
Subjects
Subjects were recruited from employees of the University Medical Center Freiburg by advertising. The study protocol was approved by our Ethics Commission. Exclusion criteria included kidney stones, pregnancy, anticonvulsant or steroid therapy in any form, frequenting a tanning salon, or residence in the mountains or southern countries right before or during the study. The subjects were not allowed to take vitamin D supplements or fish liver oils, and were asked to eat fish no more than once a week.
Caucasian adults in good general health, younger than 45 years with a body mass index between 18.5–26 kg/m2 and not fulfilling any exclusion criterion were eligible to provide blood specimens for further testing after having signed a written consent form. Out of 49 female and male volunteers, we randomized 27 subjects with low serum vitamin D (25OHD ⩽50 nmol/l) and normal serum calcium concentrations (2.2–2.7 mmol/l) to enter the study.
Study design
This study was a 5-week, prospective, randomized, 3-arm, single-blind, placebo-controlled trial to investigate the bioavailability of vitamin D2 from UV-B-irradiated button mushrooms and vitamin D2 supplement, respectively.
The four first weekly visits (weeks 0, 1, 2, 3) constituted the interventional part of the study and the two last visits (weeks 4, 5) served as follow-up. The primary objective and further endpoints were analyzed till week 4, because that is when we expected the strongest interventional effect. At the initial, baseline visit (week 0), weight and height were documented. At each subsequent weekly visit at the same time of day blood was drawn. The study was performed during the winter from late January till early March 2010, when (a) a low vitamin D status in healthy subjects is most likely and (b) solar UV-B radiation is minimal to avoid the confounding effect of cutaneous vitamin D3 synthesis on our intake-response evaluation.
Our 27 blinded subjects were randomly assigned into three equal groups ((a) mushroom, (b) supplement and (c) placebo) using a computer-generated sequence to receive four times at weekly intervals either (a) 28 000 IU vitamin D2 via 365 g of the experimental soup containing the UV-B-irradiated mushrooms (vitamin D2 content of 191.8 μg/100 g) and placebo, or (b) 60 IU vitamin D2 via a conventional mushroom soup and 28 000 IU vitamin D2 via a supplement (equivalent of 70 drops), or (c) 60 IU vitamin D2 by a conventional mushroom soup and placebo, respectively. The liquid supplement used (Stérogyl, Desma Pharma, Paris, France) provided 400 IU per drop (verified as 393 IU per drop by SGS Institut Fresenius, Berlin, Germany), consisted of an ethanol formulation of vitamin D2 and was dissolved in orange juice. The placebo consisted of pure orange juice. The supplement or placebo was served shortly before the soup. Soup intake was supervised and remains of the soup were absorbed by bread and eaten.
Blood sample analysis
The blood samples were stored for coagulation about 30–60 min in the dark at room temperature previous to centrifugation (2000 r.p.m. for 7 min). The serum samples were frozen at −78 °C until weekly analysis of all samples of one blood drawing by the laboratory MVZ Clotten (Freiburg, Germany). Serum 25OHD2 and serum 25OHD3 were measured combined as 25OHD by a radioimmunoassay purchased from DiaSorin Inc. (Stillwater, MN, USA). The quality and accuracy of the serum 25OHD analysis were monitored by interlaboratory tests evaluated by INSTANT e.V. (Duesseldorf, Germany). The detection limit for the radioimmunoassay assay was 10 nmol/l, inter- and intra-assay coefficients of variation for 25OHD were 11.1 and 10.1%. The cross-reactivity of each compound, normalized to 25OHD3 and above 1% is specified by the manufacturer as 104, 40 and 17% with 25OHD2, 1,25OH2D2, and 1,25OH2D3, respectively. Serum intact parathyroid hormone (iPTH) was measured by non-competitive immunoassay on the Roche Modular Analytics E170 (Mannheim, Germany). Serum calcium was measured using a photometric color test with Olympus calcium Arsenazo III OSR60117 (Beckman Coulter Diagnostics Ltd, O'Callaghans Mills, Ireland). The reference ranges were 50–175 nmol/l, 1.2–4.5 pmol/l and 2.2–2.7 mmol/l, for 25OHD, iPTH and serum calcium, respectively. Serum 25OHD and calcium were measured weekly; serum iPTH was measured twice at week 0 and week 4.
Irradiation of mushrooms
We used fresh brown button mushrooms (Agaricus bisporus) provided by a local mushroom producer (Schlossbergpilze, Freiburg, Germany) with a moisture content of 91.4%, determined by the vacuum oven method. To produce vitamin D2-enhanced mushrooms, they were placed completely separated from each other on a 2 cm meshed grid, and each side was irradiated simultaneously with UV-B (306 nm) at an irradiation dose of 1.5 J/cm2 after 25 min at ambient temperature (22 °C). The custom-made UV unit was equipped with 8 UV-B lamps 176 cm in length (UV21, Waldmann, Villingen-Schwenningen, Germany). The total irradiation area was 0.72 m2 with a homogeneous intensity of UV-B. The radiation dose was measured by a radiometer (UV34, PCE Group, Meschede, Germany).
Soup preparation and analytic method
Directly after irradiation, the mushrooms were diced and used in a pureed mushroom soup. All the experimental and conventional mushroom soups needed for the study were portioned out and stored in a freezer at −20 °C. Soup ingredients were water, button mushrooms, soy cream, flour, olive oil and spices (4.2% fat, 0.8% protein, 2.9% carbohydrates (weight/weight)). Soup and mushroom samples were shipped on dry ice to SGS Institut Fresenius for vitamin D2 analysis. The assay is based on semipreparative HPLC purification followed by analytical reversed-phase HPLC. Preliminary tests (data not shown) showed that the experimental soup's vitamin D2 remained very stable during cooking, freezing, defrosting and reboiling. The mushroom soups were well tolerated.
Sample size calculation and statistics
The study was designed to detect a difference of 20 nmol/l in 25OHD serum concentrations between the mushroom and placebo groups with a power of 80% with a one-sided t-test at a significance level of 5%. The assumed standard deviations were 13.0 in the placebo and 14.7 in the mushroom groups, derived from previous investigations. The resulting sample size was seven per group. The study was analyzed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) and SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
For descriptive data analyses, values are presented as means±standard deviations. Group comparisons were made using two sample t-tests and one-way analysis of variance with post hoc Tukey tests. P-values will be provided for the comparisons of secondary objectives and should be regarded as exploratory. Results with P<0.05 will be denoted as significant. The development of 25OHD concentrations during the study was investigated using a random effects model (SAS proc mixed) with 25OHD as dependent variable, different time slope parameters for each treatment group (that is time*treatment interaction) and subject specified as a random effect. Treatment differences during the study can thus be investigated by testing the differences in time slopes. Results will be given in terms of estimates for regression slope parameters, which represent the 25OHD increase per week in each group and their accompanying 95% confidence intervals (95% CIs).
Results
Baseline characteristics of subjects
Of the 27 subjects recruited for the study, one from the mushroom group dropped out because of pregnancy before the first visit, whereas 26 completed the interventional phase and were used for the further evaluation. One subject had to miss the last follow-up blood withdrawal (week 5).
Characteristics of the study population are summarized in Table 1. Mean age and body mass index of the subjects were 30.8±5.8 years and 22.1±2.5 kg/m2, respectively. There were no significant differences in these parameters among the three study groups at baseline. In addition, the three study groups were similar with regard to initial serum concentrations of 25OHD, iPTH and calcium (Table 2). At 4.08 (1.89–7.70) pmol/l at baseline, median serum iPTH was in the upper normal range, and eight subjects (30.8%) already presented secondary hyperparathyroidism as a result of vitamin D deficiency. Furthermore, we found a statistically significant negative linear correlation between iPTH and serum 25OHD (r=−0.463, P=0.017).
Full size table
Full size table
Mushrooms' vitamin D2 content
Our study's brown button mushrooms cultivated in the dark had very low concentrations of vitamin D2 (0.18 μg/100 g fresh weight). The conversion of ergosterol to vitamin D2 under UV-B irradiation in this study was very high and we achieved concentrations of 491 μg/100 g fresh weight (56.8 μg vitamin D2/g dry solids).
Time course of the main serum parameters
The time course data of serum 25OHD concentrations for each study group over the 5-week period are presented in Table 2 and Figure 1.
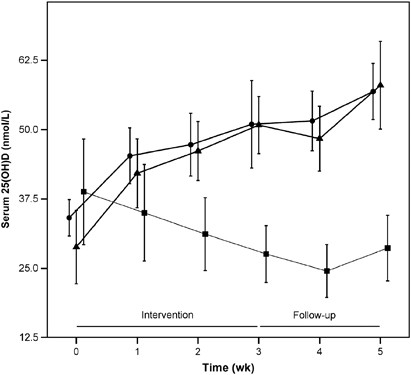
Time course of the mean changes in serum 25OHD over the 5-week study period in subjects who consumed four times (weeks 0, 1, 2, 3) mushrooms enhanced with vitamin D2 via UV-B irradiation (mushroom group, n=8, •) or vitamin D2-containing supplements (supplement group, n=9, ▴) or placebo (placebo group, n=9, ▪) at the end of the winter. Error bars are 2 s.e. At week 5, one subject dropped out of the supplement group. Concentrations were significantly different (analysis of variance, Tukey's test) between the mushroom and placebo groups, and between the supplement and placebo groups from week 2 onward (wk 2: P=0.002; P=0.004, respectively; weeks 3, 4, 5: P<0.0001). Time courses for serum 25OHD over the study period in the mushroom and supplement groups did not differ significantly.
Full size image
The primary objective was to test the efficacy of the vitamin D2-enhanced mushrooms to improve the 25OHD status. The data show that 1 week after the last consumption of such mushrooms at week 4, the mushroom group's serum 25OHD was significantly higher than that in the placebo group (P<0.0001) (Table 2).
A secondary objective consisted of testing the bioavailability of vitamin D2 from the UV-B treated mushrooms compared with a common vitamin D2 supplement. When modeling the development of 25OHD concentrations as a linear function of time (mixed regression model), we found that the mushroom and supplement groups increased their 25OHD concentrations significantly over the study period by 3.9 nmol/l per week (95% CI: 2.9, 4.8; P<0.0001) and by 4.7 nmol/l per week (95% CI: 3.8, 5.7; P<0.0001) (Figure 1). Regression slopes of the concentrations of the serum 25OHD in the mushroom and supplement groups did not significantly differ from one another (P=0.20). The mean increase in serum 25OHD in the first 4 weeks per 100 IU of vitamin D2 was 0.5 nmol/l.
Further analysis showed that already 2 weeks after the first consumption of the vitamin D2-enhanced mushrooms, the two interventional groups' serum 25OHD concentrations were significantly higher than in the placebo group (P=0.001) (Table 2).
Furthermore, the data reveal significant within-subject changes in serum 25OHD in all three study groups already at week 1. During the first week, serum 25OHD rose significantly (P<0.001) by 33.1 and 46.1% in the mushroom and supplement groups, respectively. In addition, serum 25OHD decreased significantly (P=0.03) by −6.5% in the placebo group. The development of hypercalcaemia (serum calcium >2.7 mmol/l) was the main safety criteria for the vitamin D2 administration. Serum calcium remained within the reference range at all time points. No physical symptoms were reported during the study.
Neither at baseline nor week 4 did the serum concentrations in iPTH and calcium differ significantly among the three study groups (Table 2). The correlation between the 4-week changes in both intervention groups from serum 25OHD and iPTH at baseline revealed a negative but nonsignificant association (r=−0.449, P=0.071).
Discussion
Here we describe for the first time in humans that UV irradiation of mushrooms creates an excellent source of vitamin D2, which has equivalent bioavailability as a vitamin D2 supplement.
The rapid serum 25OHD increase in our mushroom group is a clear demonstration that ingesting 28 000 IU vitamin D2 once a week for 4 weeks via UV-B-irradiated and vitamin D2-enhanced mushrooms is effective in improving vitamin D status in young, healthy adults. Already 1 week after their first ingestion of UV-B-irradiated mushrooms, serum 25OHD rose significantly. Furthermore, it was significantly higher than in placebo group 1 week after the second administration of enhanced mushrooms (week 2).
Most studies on the bioavailability of vitamin D in humans have been conducted using supplements, not natural food sources. Consistent with our observation of equivalent vitamin D2 bioavailability from a soup prepared with UV-irradiated mushrooms and supplement, an earlier study (Outila et al., 1999) demonstrated the same efficiency with non-irradiated, but lyophilized and homogenized mushrooms in humans. Both findings disprove the hypothesis of van den Berg (1997), who maintained that the bioavailability of vitamin D from natural food sources is probably lower than from supplements.
By our weekly vitamin D2 supplementation of 28 000 IU (daily equivalent of 4000 IU), we found after 1 month a mean increase in serum 25OHD of 0.5 nmol/l for every 100 IU of vitamin D ingested. Evaluating our dose-response effect with the reported increase by 1–2 nmol/l in 25OHD for each additional 100 IU of vitamin D3 (Cranney et al., 2008) is not appropriate, because a plateau in serum 25OHD was not achievable over such a short period. Therefore, we examined supplementation studies using comparable doses of vitamin D2 and time periods. Mastaglia et al. (2006) reported a dose-response in the first month of 0.43 nmol/l for every 100 IU vitamin D2 by supplementing weekly 35 000 IU of vitamin D2, resembling our results although they used a 25% higher dose. At their study's conclusion (3 months), dose-response rose to 0.71 nmol/l. Considering the differences in doses and time, our results also concur with dose-responses reported by others (Malabanan et al., 1998; Pietras et al., 2009).
One of our study's limitations is that the accuracy of the radioimmunoassay method used to determine 25OHD2 was found to be lower than indicated by the manufacturer (Glendenning et al., 2003; Glendenning et al., 2006), so it is possible that total serum 25OHD was underestimated.
Other authors showed that a single dose of 50 000 IU of vitamin D2 or vitamin D3 produced similar increases in serum 25OHD over the first 3 days, but serum 25OHD began to fall immediately thereafter in the vitamin D2 group until, by day 14, it reached baseline concentrations (Armas et al., 2004). In contrast, serum 25OHD concentrations continued to rise until day 14 in the vitamin D3 group, then fell slowly over the following 14 days.
We did not compare the potencies of these two types of vitamin D and could not confirm the reported short initial increase followed by a rapid fall in serum 25OHD after vitamin D2 supplementation (Armas et al., 2004). When adjusted for a concomitant increase in serum 25OHD in the placebo group in the last week (due to the confounding effect of cutaneous vitamin D3 synthesis), the high serum 25OHD concentrations in both interventional groups achieved remained constant during the follow-up period.
Secondary hyperparathyroidism at baseline as a major clinical sign of vitamin D deficiency was present in almost a third of our subjects with low 25OHD status, especially in those with very low serum 25OHD concentrations (⩽25 nmol/l). In addition, we observed a negative correlation (r=−0.449, P=0.071) between serum 25OHD increase due to the weekly intake of 28 000 IU vitamin D2 and the change in serum iPTH.
As anticipated, and in light of the current state of knowledge (Vieth et al., 2001; Hathcock et al., 2007), the vitamin D2 dose of an average of 4000 IU/d used in this study was safe, and we observed no cases of hypercalcaemia or any adverse events.
Our results demonstrate for the first time that the bioavailability of vitamin D2 from vitamin D2-enhanced button mushrooms via UV-B irradiation was effective in improving vitamin D status in young, healthy adults. Furthermore, we did not observe any differences in the absorption rate and metabolism of vitamin D2 from UV-B-irradiated mushrooms and a vitamin D2 supplement in raising circulating serum 25OHD concentrations.
In conclusion, as the vitamin D2 enhancement of mushrooms boosts their nutraceutical value, it would provide a worthwhile means of improving the vitamin D maintenance in the general population. Further research and development are required to find solutions for making such vitamin D2-enhanced mushrooms commercially available in a safe and affordable manner.
References
-
Armas LA, Dowell S, Akhter M, Duthuluru S, Huerter C, Hollis BW et al. (2007). Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol 57, 588–593.
PubMed Article Google Scholar
-
Armas LA, Hollis BW, Heaney RP (2004). Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab 89, 5387–5391.
CAS PubMed Article Google Scholar
-
Biancuzzo RM, Young A, Bibuld D, Cai MH, Winter MR, Klein EK et al. (2010). Fortification of orange juice with vitamin D(2) or vitamin D(3) is as effective as an oral supplement in maintaining vitamin D status in adults. Am J Clin Nutr 91, 1621–1626.
CAS PubMed PubMed Central Article Google Scholar
-
Calvo MS, Whiting SJ, Barton CN (2005). Vitamin D intake: a global perspective of current status. J Nutr 135, 310–316.
CAS PubMed PubMed Central Article Google Scholar
-
Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S et al. (1997). Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int 7, 439–443.
CAS PubMed PubMed Central Article Google Scholar
-
Clemens TL, Adams JS, Henderson SL, Holick MF (1982). Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet 1, 74–76.
CAS PubMed Article Google Scholar
-
Cranney A, Weiler HA, O'Donnell S, Puil L (2008). Summary of evidence-based review on vitamin D efficacy and safety in relation to bone health. Am J Clin Nutr 88, 513S–519S.
CAS PubMed Article Google Scholar
-
Engelsen O, Brustad M, Aksnes L, Lund E (2005). Daily duration of vitamin D synthesis in human skin with relation to latitude, total ozone, altitude, ground cover, aerosols and cloud thickness. Photochem Photobiol 81, 1287–1290.
CAS PubMed Article Google Scholar
-
Glendenning P, Noble JM, Taranto M, Musk AA, McGuiness M, Goldswain PR et al. (2003). Issues of methodology, standardization and metabolite recognition for 25-hydroxyvitamin D when comparing the DiaSorin radioimmunoassay and the Nichols advantage automated chemiluminescence protein-binding assay in hip fracture cases. Ann Clin Biochem 40, 546–551.
CAS PubMed Article Google Scholar
-
Glendenning P, Taranto M, Noble JM, Musk AA, Hammond C, Goldswain PR et al. (2006). Current assays overestimate 25-hydroxyvitamin D3 and underestimate 25-hydroxyvitamin D2 compared with HPLC: need for assay-specific decision limits and metabolite-specific assays. Ann Clin Biochem 43, 23–30.
CAS PubMed Article Google Scholar
-
Hathcock JN, Shao A, Vieth R, Heaney R (2007). Risk assessment for vitamin D. Am J Clin Nutr 85, 6–18.
CAS PubMed Article Google Scholar
-
Hintzpeter B, Mensink GB, Thierfelder W, Muller MJ, Scheidt-Nave C (2008). Vitamin D status and health correlates among German adults. Eur J Clin Nutr 62, 1079–1089.
CAS PubMed Article Google Scholar
-
Holick MF, Chen TC (2008). Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 87, 1080S–1086S.
CAS Article Google Scholar
-
Holick MF, Shao Q, Liu WW, Chen TC (1992). The vitamin D content of fortified milk and infant formula. N Engl J Med 326, 1178–1181.
CAS PubMed Article Google Scholar
-
Jasinghe VJ, Perera CO (2005). Distribution of ergosterol in different tissues of mushrooms and its effect on the conversion of ergosterol to vitamin D2 by UV irradiation. Food Chem 92, 541–546.
CAS Article Google Scholar
-
Jasinghe VJ, Perera CO (2006). Ultraviolet irradiation: the generator of vitamin D2 in edible mushrooms. Food Chem 95, 638–643.
CAS Article Google Scholar
-
Ko JA, Lee BH, Lee JS, Park HJ (2008). Effect of UV-B exposure on the concentration of vitamin D2 in sliced shiitake mushroom (Lentinus edodes) and white button mushroom (Agaricus bisporus). J Agric Food Chem 56, 3671–3674.
CAS PubMed Article Google Scholar
-
Koyyalamudi SR, Jeong SC, Song CH, Cho KY, Pang G (2009). Vitamin D2 formation and bioavailability from Agaricus bisporus button mushrooms treated with ultraviolet irradiation. J Agric Food Chem 57, 3351–3355.
CAS PubMed Article Google Scholar
-
Malabanan A, Veronikis IE, Holick MF (1998). Redefining vitamin D insufficiency. Lancet 351, 805–806.
CAS Article Google Scholar
-
Mastaglia SR, Mautalen CA, Parisi MS, Oliveri B (2006). Vitamin D2 dose required to rapidly increase 25OHD levels in osteoporotic women. Eur J Clin Nutr 60, 681–687.
CAS PubMed Article Google Scholar
-
Mattila P, Lampi AM, Ronkainen R, Toivo J, Piironen V (2002). Sterol and vitamin D2 contents in some wild and cultivated mushrooms. Food Chem 76, 293–298.
CAS Article Google Scholar
-
Mau JL, Chen PR, Yang JH (1998). Ultraviolet irradiation increased vitamin D2 content in edible mushrooms. J Agric Food Chem 46, 5269–5272.
CAS Article Google Scholar
-
Natri AM, Salo P, Vikstedt T, Palssa A, Huttunen M, Karkkainen MU et al. (2006). Bread fortified with cholecalciferol increases the serum 25-hydroxyvitamin D concentration in women as effectively as a cholecalciferol supplement. J Nutr 136, 123–127.
CAS PubMed Article Google Scholar
-
Need AG, Morris HA, Horowitz M, Nordin C (1993). Effects of skin thickness, age, body fat, and sunlight on serum 25-hydroxyvitamin D. Am J Clin Nutr 58, 882–885.
CAS PubMed PubMed Central Article Google Scholar
-
Outila TA, Mattila PH, Piironen VI, Lamberg-Allardt CJ (1999). Bioavailability of vitamin D from wild edible mushrooms (Cantharellus tubaeformis) as measured with a human bioassay. Am J Clin Nutr 69, 95–98.
CAS PubMed Article Google Scholar
-
Ozzard A, Hear G, Morrison G, Hoskin M (2008). Vitamin D deficiency treated by consuming UVB-irradiated mushrooms. Br J Gen Pract 58, 644–645.
PubMed PubMed Central Article Google Scholar
-
Pietras SM, Obayan BK, Cai MH, Holick MF (2009). Vitamin D2 treatment for vitamin D deficiency and insufficiency for up to 6 years. Arch Intern Med 169, 1806–1808.
PubMed Article Google Scholar
-
Roberts JS, Teichert A, McHugh TH (2008). Vitamin D2 formation from post-harvest UV-B treatment of mushrooms (Agaricus bisporus) and retention during storage. J Agric Food Chem 56, 4541–4544.
CAS PubMed Article Google Scholar
-
Semba RD, Garrett E, Johnson BA, Guralnik JM, Fried LP (2000). Vitamin D deficiency among older women with and without disability. Am J Clin Nutr 72, 1529–1534.
CAS PubMed PubMed Central Article Google Scholar
-
Tangpricha V, Pearce EN, Chen TC, Holick MF (2002). Vitamin D insufficiency among free-living healthy young adults. Am J Med 112, 659–662.
CAS PubMed PubMed Central Article Google Scholar
-
van den Berg H (1997). Bioavailability of vitamin D. Eur J Clin Nutr 51(Suppl 1), S76–S79.
PubMed Google Scholar
-
van der Wielen RP, Lowik MR, van den Berg H, de Groot LC, Haller J, Moreiras O et al. (1995). Serum vitamin D concentrations among elderly people in Europe. Lancet 346, 207–210.
CAS PubMed Article Google Scholar
-
Vieth R, Chan PC, MacFarlane GD (2001). Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr 73, 288–294.
CAS PubMed Article Google Scholar
-
Webb AR, Kline L, Holick MF (1988). Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 67, 373–378.
CAS PubMed PubMed Central Article Google Scholar
Download references
Acknowledgements
We are grateful to Carole Cürten for proofreading this manuscript, to Peter Metzger from Schlossbergpilze, Freiburg, Germany, for donating the button mushrooms used in this study, to Daniela Klein and Elmar Maier for their help preparing the soups in the university's kitchen and to Weber GmbH for technical support with the construction of the UV unit. The authors' responsibilities were as follows: PU, study concept and design, obtaining funding, statistical analysis, data interpretation and writing the manuscript; FS, construction of UV-unit, collection, assembly and interpretation of data; GI, study design, statistical advice and analysis; HKB, critical review and contribution to the final draft; HB, supervision of the study, critical review and contribution to the final draft. All authors have read and approved the final manuscript.
The work was supported by a PhD grant (PU) from the National Research Fund, Luxemburg. Financial support for the irradiation experiments was by the Dr Heinrich-Kircher Foundation, University of Freiburg, Freiburg, Germany.
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Urbain, P., Singler, F., Ihorst, G. et al. Bioavailability of vitamin D2 from UV-B-irradiated button mushrooms in healthy adults deficient in serum 25-hydroxyvitamin D: a randomized controlled trial. Eur J Clin Nutr 65, 965–971 (2011). https://doi.org/10.1038/ejcn.2011.53
Download citation
-
Received:
-
Revised:
-
Accepted:
-
Published:
-
Issue Date:
-
DOI : https://doi.org/10.1038/ejcn.2011.53
Keywords
- vitamin D2
- ergosterol
- button mushrooms
- ultraviolet irradiation
- bioavailability
- humans
Further reading
Source: https://www.nature.com/articles/ejcn201153

0 Komentar